Bard Power Port Lawsuit | Injuries from Defective Catheters
In the world of medical advancements, Bard Power Port has become both a lifesaver and a cause for concern. Specifically designed for treatments like chemotherapy, this implantable, intravenous catheter has found its way into critical healthcare procedures. However, beneath its life-saving potential lies a web of complexity and controversy, leading many to consider the path of the Bard Power Port lawsuit.
In this guide, Ethen Ostroff Law delves into the intricacies of Bard Power Port, from its various types to the prevalent issues affecting patients. We explore alleged defects, understanding the complications they may cause. We also navigate the legal landscape, breaking down the Bard Power Port lawsuit process. Finally, we outline who can file a Bard PowerPort lawsuit, identify potential defendants, and explain the claims and damages that can be pursued. Join us as we simplify the complexities, empowering you with the knowledge to make informed decisions and take confident steps toward seeking justice.
Introducing Bard PowerPort
The Bard PowerPort isn’t just a medical device; it’s a lifeline for those undergoing challenging treatments. About the size of a quarter, this implant sits discreetly under the skin on the chest or arm, providing direct access to a vein for medications, fluids, and more. Think of it as a reliable companion during medical IV therapy, chemotherapy, and other therapies for conditions like chronic kidney disease. It’s designed to make life a bit easier, offering a safe and convenient solution that minimizes the discomfort of repeated needle sticks.
PowerPort Manufacturer and FDA Approval
PowerPort is manufactured by Bard Access Systems, Inc., a part of the Becton, Dickinson, and Company (BD) family. Specifically, it’s born from the expertise of the C. R. Bard division, known for creating vascular access solutions like catheters for dialysis and chemotherapy. While C. R. Bard used to be a standalone company, it became part of BD in 2017. Bard PowerPort’s approval from the U.S. Food and Drug Administration (FDA) came in 2000, ensuring that patients needing frequent medications, IV fluids, blood products, and nutrition solutions have a trusted, vetted option for their long-term access needs.
Bard PowerPort Device Design
Bard PowerPort is a thoughtful design—gentle, effective, and makes one’s health experience a bit more compassionate. Let’s break down the Bard PowerPort into two crucial parts:
- The injection port: It is a small, self-sealing port beneath your skin, like a health ally. With a silicone septum, a needle can pass through, delivering medication straight into the reservoir for treatments like IV therapy or blood transfusion.
- The catheter: It is a flexible tube linking the injection port to a vein. It’s made of polyurethane, or in some cases, a strong material called ChronoFlex. This material makes it durable, ensuring reliability for your healthy journey.
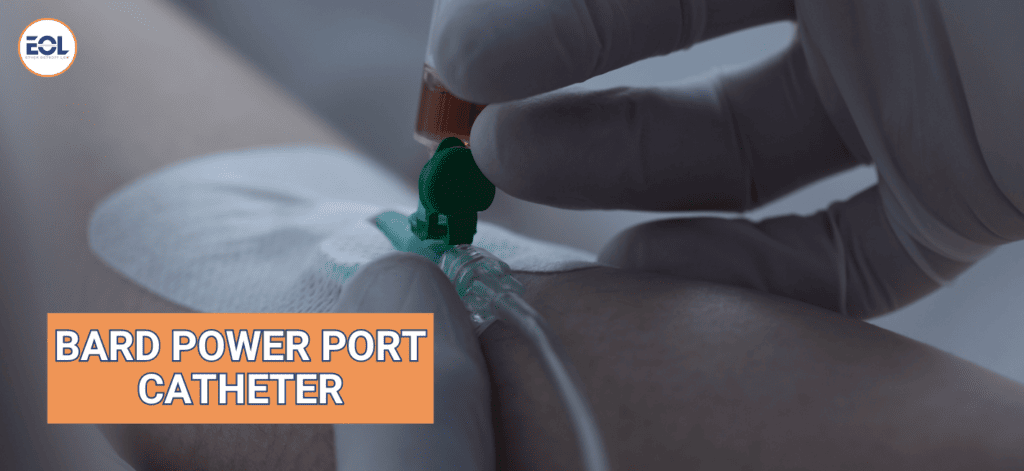
Process of Implanting Bard PowerPort
When a Bard PowerPort is implanted, it’s a careful procedure. The port is inserted into an opening on your chest. The doctor gently tunnels the catheter, a small, soft tube under your skin, towards the base of your neck and into your vein. To ensure everything is working correctly, a specialized port needle is used to access the port.
How the Bard PowerPort Works
The Bard PowerPort is a subcutaneous, implantable port catheter system that delivers medications such as blood products, chemotherapy drugs, intravenous fluids, and parenteral nutrition solutions. The device is made up of an injection port and a catheter that serves as a conduit for intravenous therapies. The catheter tip is advanced from its insertion point to the junction of the superior vena cava and the right atrium, where medication or fluids are injected into the bloodstream. To enter the bloodstream, medical professionals must use the PowerLoc needle, which ensures a secure and efficient connection to the PowerPort.
Bard PowerPort Options
Bard PowerPort comes in various configurations to cater to different medical needs, namely:
- PowerPort™ ClearVUE™ Implantable Ports
- PowerPort™ duo M.R.I.™ Implantable Port
- PowerPort™ Implantable Port
- PowerPort™ ISP Implantable Port
- PowerPort™ M.R.I.™ Implantable Port
These PowerPort devices have their own unique materials, designs, and features. Made from materials like polymer, polyurethane, silicone, and titanium, each is designed with options like low-profile, ultra-slim, and dual-lumen. From radiopacity to MRI compatibility and attachable catheters, Bard PowerPort devices are here to cater to your individual medical needs, ensuring your journey is as comfortable as possible.
Bard PowerPort Design Flaws
Despite its intended purpose, Bard PowerPort’s design and manufacturing flaws have been associated with various device failures. Allegations of defects in Bard PowerPort include issues with the device’s materials, design flaws, and malfunctions that can compromise its functionality. The design flaws and manufacturing issues with the Bard PowerPort have made the device prone to these three primary complications or post-implantation failures:
- Catheter fracture injuries: Bard PowerPort devices use materials like Chronoflex with questionable mechanical integrity, such as polyurethane and barium sulfate, which can make the catheter tubes prone to fissuring, cracking, and fracturing. Device fractures pose a particularly significant risk. This failure mode involves the catheter component of the PowerPort breaking apart, with fragments potentially traveling through the patient’s bloodstream. These fragments can cause severe and often fatal conditions. To remove fractured pieces and treat affected organ systems, emergency surgery is frequently required.
- Catheter migration: Another major complication is device migration. The PowerPort device moves from its original location within the patient’s body. This movement can cause serious internal damage, especially if the device comes into contact with vital organs or tissues. Furthermore, if the device migrates, it may lose effectiveness or become completely inoperable, disrupting the patient’s treatment schedule.
- Catheter-related infections: The third major complication of this device is serious infections that start at the port site. The flawed nature of the material used to make the Bard PowerPort does not prevent bacteria from growing, especially when the material fractures or degrades.
Complications and Injuries
The design flaws of Bard PowerPort devices have led to various severe complications and injuries
including:
- Allergic reaction
- Bloody cough
- Brachial plexus injury
- Cardiac arrhythmia
- Cardiac punctures
- Cardiac tamponade
- Catheter or port erosion through the skin
- Catheter migration
- Catheter fracture
- Chest pain
- Chronic pain
- Confusion
- Death
- Embolisms (pulmonary, air)
- Fever
- Headaches
- Hematomas
- Hemorrhage
- Incorrect/insufficient delivery of essential fluids or medication
- Infections (sepsis)
- Lacerated blood vessels
- Necrosis (death of body tissue)
- Organ or tissue perforation (heart or lungs)
- Pinch-off syndrome
- Restricted blood flow
- Shortness of breath
- Thromboembolism (blood clots)
- Urinary changes
- Vital organ damage
Understanding these complications is crucial for those affected and their legal representation.
Bard PowerPort Recall
Though hundreds of serious adverse event reports have been filed with the FDA due to fragmentation and infection issues, Bard has not recalled any PowerPort catheters because of these injuries and continues to manufacture and market the devices. A previous Class II recall was issued in March 2020 by the FDA. It was for a small number of Bard PowerPorts which may have contained the wrong type of tubing tip. This recall had nothing to do with the risk of fragmentation, infection, or any injuries.
Bard PowerPort Lawsuits
The design flaws and resulting complications linked to PowerPort have sparked a series of lawsuits against Bard. Individuals who have faced issues, complications, or harm with Bard PowerPort devices are taking legal action. These lawsuits are a pursuit of compensation for the challenges tied to the device.
Allegations in PowerPort Lawsuits
The allegations against Bard in PowerPort lawsuits include:
- Concealing multiple reports of PowerPort failure through the FDA Alternative Summary Reporting program.
- Deliberate disregard for the potential harm they were causing.
- Failing to adequately disclose the risk of port catheter complications associated with the products to patients or the medical community.
- Failing to conduct sufficient post-marketing surveillance to better identify reports of injury and death.
- Failing to improve the PowerPort or issue a recall.
- Hiding the severity of PowerPort-related complications.
- PowerPort devices are defective and dangerous.
Several implantable catheter ports have been named in Bard port lawsuits, including:
- PowerPort™ ClearVUE™ Implantable Port
- PowerPort™ ClearVUE™ ISP Implantable Port
- PowerPort™ ClearVUE™ Slim Implantable Port
- PowerPort™ isp M.R.I.™ Implantable Port
- PowerPort™ M.R.I.™ Implantable Port
Defendants in PowerPort Lawsuits
Defendants in the port catheter lawsuits include the following corporations involved in the design and sale of PowerPort implants:
- Bard Access Systems, Inc.
- Becton, Dickinson and Company
- C.R. Bard
Legal Claims in PowerPort Lawsuits
In Bard Power Port lawsuit, people are seeking compensation for the harm they’ve endured. Here are the main legal claims:
- Breach of warranty: Saying Bard broke its promise, delivering a defective or substandard Bard PowerPort.
- Failure to warn: Claiming Bard didn’t give enough warnings about potential risks, causing unforeseen harm.
- Loss of consortium: Sometimes, family members file for the loss of companionship due to Bard PowerPort injuries.
- Medical negligence: If healthcare professionals are involved, individuals might file claims for negligent implantation or monitoring of the Bard PowerPort.
- Negligence: Accusing Bard of negligence in designing, making, or distributing the Bard PowerPort.
- Pain and suffering: Seeking compensation for physical and emotional distress from Bard PowerPort complications.
- Personal injury: Claiming personal injury from Bard PowerPort issues like infections, device migration, or damage to surrounding tissues.
- Product liability: Alleging that the Bard PowerPort is defective or unreasonably dangerous under product liability laws, covering design, manufacturing, or warning defects.
These claims are the foundation of Bard Power Port lawsuit. Product liability and defective medical device attorneys, such as those at Ethen Ostroff Law, play a crucial role in guiding individuals through the legal process and building a robust case to support these claims.
MDL 3081
Because many lawsuits have been filed against the makers of Bard PowerPort, a multi-district litigation (MDL) has been set up in the District of Arizona to consolidate all the federal lawsuits about injuries caused by Bard PowerPorts. MDL 3081 (IN RE: Bard Implanted Port Catheter Products Liability Litigation) started in 2018 in the Southern District of West Virginia. It aims to combine all Bard PowerPort lawsuits against Bard, claiming these devices are defective and dangerous. The goal of the MDL is to make the early legal steps more efficient, reduce costs and time, and prevent conflicting decisions. On August 8, 2023, the U.S. Judicial Panel on Multidistrict Litigation decided to move all claims to the District of Arizona under U.S. District Court Judge David G. Campbell for coordinated pretrial proceedings.
Bard Power Port Lawsuit Updates
- October 25, 2023: The Bard PowerPort product liability lawsuits in federal courts are now consolidated into a class action MDL, with 62 pending cases. In addition, another class action MCL (multi-county litigation) is being pursued to consolidate over 500 PowerPort lawsuits in New Jersey state courts.
- October 16, 2023: Twelve new cases added to the Bard PowerPort class action MDL in the last 30 days, bringing the total pending cases to 62.
- October 11, 2023: A recent Bard PowerPort lawsuit was filed by a Chicago woman. Her PowerPort, inserted for breast cancer chemo in 2020, fractured in less than a year, and fragments ended up in her heart. She filed her lawsuit in federal court in Chicago.
- October 2, 2023: Judge David G. Campbell, overseeing the Bard PowerPort class action MDL, issued Case Management Orders outlining the MDL’s future direction.
- September 18, 2023: Last month, 40 new cases were transferred into the Bard PowerPort MDL, bringing the total pending cases to 50.
- August 18, 2023: The newly formed MDL class action lawsuit involving alleged defects with the Bard PowerPort has 10 pending cases.
- August 12, 2023: A class action for Bard Power Port lawsuit is established in federal court in Arizona, simplifying pretrial proceedings and potentially expediting a settlement.
- July 25, 2023: The JPML holds a hearing to decide whether all federal Bard Power Port lawsuits should be combined for streamlined discovery and pretrial proceedings in an MDL.
- June 21, 2023: Despite numerous Bard Power Port lawsuits alleging severe problems, the manufacturer contests efforts to unify claims under one judge. Lawsuits assert inherent defects causing fractures, infections, blood clots, and more.
- June 8, 2023: A motion is filed with the U.S. Judicial Panel on Multidistrict Litigation (JPML) to consolidate all Bard PowerPort product liability lawsuits in federal courts into a new class action MDL. Presently, there are 10 lawsuits, with an expected significant increase in the next year.
Process for Filing Bard Power Port Lawsuit
Individuals considering filing a Bard Power Port lawsuit need to be aware of the procedural aspects involved. From gathering evidence to filing the necessary documents, here are the steps to initiating legal action:
- Consultation with an attorney: Talk to a lawyer about your Bard PowerPort issues. They’ll review your case and advise if it has legal merit.
- Case evaluation: The lawyer will examine your medical records and evidence to assess the strength of your case.
- Confirmation of eligibility: Ensure you meet the criteria for filing a lawsuit by discussing your situation thoroughly with your lawyer.
- Gather evidence and documentation: Collect the required evidence and documentation, including medical records, test results, communication records, documentation of complications, and witness testimony.
- Filing the lawsuit: Your lawyer will handle the paperwork to officially start legal proceedings against Bard, the manufacturer.
- MDL or individual lawsuit: Your case may join others in an MDL or proceed as an individual lawsuit, depending on the circumstances.
- Discovery process: Both sides exchange information and evidence as part of the legal process.
- Settlement negotiations: Your lawyer may negotiate with Bard to settle before going to trial.
- Trial preparation: If a settlement is unlikely, your lawyer will prepare for trial, gathering evidence and identifying witnesses.
- Trial: Present your case in court before a judge or jury.
- Verdict and damages: After the trial, if successful, you may receive compensation for various damages, such as medical expenses or lost wages.
Statutes of Limitations for PowerPort Lawsuits
Understanding the time limits, or statutes of limitations, for filing a Bard PowerPort lawsuit is crucial. These laws, varying by location and case details, set a timeframe after an injury within which you must file a legal claim. Missing this deadline could result in losing the right to seek legal action. The statutes generally range from one to several years, depending on your jurisdiction. Filing your Bard PowerPort lawsuit promptly is vital to preserve your right to potential compensation. Consult with an attorney promptly to determine your specific timeframe and file within it.
Eligibility for Filing PowerPort Lawsuit
If you’ve faced issues with Bard PowerPort devices, you may be eligible to file a lawsuit if:
- You’ve used specific Bard PowerPort devices.
- You’ve experienced complications directly linked to the Bard PowerPort.
Eligibility criteria might differ by law firm or attorney. To understand if you qualify, it’s essential to consult with a compassionate attorney experienced in Bard PowerPort cases. They can assess your situation and guide you on available legal options.
Damages in PowerPort Lawsuits
The damages in Bard PowerPort lawsuits may include both economic and non-economic losses that an individual has incurred as a direct result of a defective Bard PowerPort device. The potential damages in a Bard PowerPort lawsuit may include:
- Medical expenses. This may include the cost of medical treatment, hospitalization, medication, and rehabilitation related to the Bard PowerPort complications.
- Lost wages. This may include the income lost due to the Bard PowerPort complications, including time off work for medical treatment and recovery.
- Pain and suffering: This may include the physical and emotional pain and suffering caused by the Bard PowerPort complications, including the loss of enjoyment of life.
- Wrongful death. In cases where an individual has died due to Bard PowerPort complications, surviving family members may be eligible to file a wrongful death lawsuit to seek compensation for their losses.
The actual damages awarded in a Bard PowerPort lawsuit may vary based on the individual facts and circumstances of each case. The potential range of settlements and payouts can be wide, influenced by factors such as the claim’s potential success in a trial and the number of damages a jury may award.
Potential Settlement Amount for a Bard Port Lawsuit
The possible settlement in a Bard Port lawsuit varies based on your specific damages and how the case unfolds. There aren’t publicized settlement amounts, and no case has reached a jury. Attorneys suggest potential settlements might range from $10,000 to over $100,000, with averages falling between $10,000 and over $250,000. Your unique situation and the course of the lawsuit will influence the potential settlement amount. It’s a sensitive process, and your attorney can provide more personalized insights based on your circumstances.
Success Rate of PowerPort Lawsuits
The success of a Bard PowerPort lawsuit is determined by the facts and circumstances of each case, such as the strength of the evidence, the severity of the injuries, and the legal representation’s skill. A thorough and well-documented claim increases the chances of success in a Bard PowerPort lawsuit. The range of potential settlements and payments can be wide, influenced by factors such as the claim’s potential success in a trial and the number of damages a jury may award. While no typical Bard PowerPort settlement amount or payout for Bard PowerPort lawsuits has been disclosed, previous legal actions against Bard have resulted in substantial awards. It is important to note that there have not been any publicly disclosed Bard PowerPort settlements, and no individual case has gone before a jury.
Is there a Bard PowerPort class action lawsuit?
As of now, there isn’t a collective Bard PowerPort class action lawsuit for those who suffered injuries or lost a loved one due to the catheter. Each person’s situation is unique, so these claims are handled individually. People affected will hire their own Bard PowerPort lawyer to investigate and file a lawsuit, showing how their specific issues resulted from the Bard Port catheter.
In the future, there might be a Bard PowerPort class action for those who received the catheter but haven’t faced any issues yet. In such a case, the damages sought could include medical monitoring, refunds, or compensation for catheter-related costs. Your attorney can provide personalized guidance based on your circumstances.
The Importance of Hiring a Bard PowerPort Lawyer
Engaging a Bard PowerPort lawyer is crucial for those seeking compensation and navigating the complexities of Bard PowerPort lawsuits. Here’s why they matter:
- Consultation: The first step in filing a port catheter lawsuit is to consult with an attorney specializing in medical device litigation. They evaluate your case, gauge its strength, and guide you through the legal process.
- Expertise: Bard PowerPort lawsuit lawyers possess expertise in personal injury, medical devices, and defective device lawsuits, ensuring adept navigation through the intricate legal aspects of Bard PowerPort cases.
- Legal representation: They can provide legal representation for individuals who have experienced complications and injuries related to Bard PowerPort devices, helping them seek compensation for their losses.
- Eligibility determination: They can help individuals determine their eligibility for filing a Bard PowerPort lawsuit and provide guidance on the legal options available to them.
- Evidence gathering: Bard PowerPort lawsuit lawyers can help individuals gather the necessary evidence and documentation to support their claim, which can increase the likelihood of a successful outcome in a Bard PowerPort lawsuit.
- Settlement negotiations: They can help individuals negotiate a settlement with the manufacturer or pursue the case through the legal system, which may involve pretrial proceedings, discovery, and settlement negotiations.
- Maximizing compensation: They can help individuals maximize their compensation by calculating and determining the unique damages in their case.
Hire Ethen Ostroff Law
In the realm of medical breakthroughs, the Bard PowerPort stands as both a medical marvel and a source of growing concern. Designed to streamline vital treatments like chemotherapy, this implantable device has found its way to the forefront of modern healthcare. However, beneath the surface of its life-saving capabilities lies a narrative of complexity and controversy that has led individuals to the doorstep of legal recourse.
Amidst the complexities of Bard PowerPort lawsuits, Ethen Ostroff Law stands as a dedicated advocate for those seeking justice. With a track record of representing individuals affected by defective medical devices, the firm brings a wealth of experience and expertise to the forefront. Ethen Ostroff Law stands as a beacon of support for those navigating the challenges posed by Bard PowerPort. We are your ally in understanding and navigating the challenges posed by the Bard PowerPort Lawsuit.


