SIRVA Lawsuit | National Vaccine Injury Compensation Program (VICP)
Vaccines, a crucial medical advancement, have safeguarded countless lives against various diseases. Yet, despite their benefits, some may experience adverse reactions or injuries. Recognizing the need to address vaccine-related injuries, the National Vaccine Injury Compensation Program (VICP) was created to compensate eligible individuals. At Ethen Ostroff Law, we explore Shoulder Injury Related to Vaccine Administration (SIRVA), SIRVA injury claims, and the VICP. We also examine the complexities of a SIRVA lawsuit and highlight how our firm can ensure your SIRVA compensation or other vaccine-related injury claim.
Vaccines and Public Health
Vaccines are crucial for public health, shielding individuals and communities from infectious diseases by boosting the body’s immune response. They curb disease spread and minimize outbreaks. However, vaccines, while greatly beneficial, can occasionally cause adverse reactions or injuries. One such example is SIRVA, resulting from improper vaccine administration. Though rare, SIRVA highlights the significance of adhering to correct vaccine administration protocols and maintaining vigilance in vaccine safety monitoring.
Understanding SIRVA
SIRVA is a distinct type of vaccine injury. It happens when a vaccine is not correctly administered, leading to shoulder pain and restricted movement. SIRVA occurs when the injection goes into the shoulder muscle instead of the intended deltoid muscle, causing inflammation and tissue damage.
How SIRVA Happens
SIRVA occurs due to improper vaccine administration, resulting in shoulder injuries. Normally, vaccines are injected into the deltoid muscle in the upper arm, stimulating the immune system without causing harm. However, in SIRVA cases, the vaccine is injected too high or too deeply into the shoulder, missing the deltoid muscle and hitting surrounding tissues like the bursa and tendons. This incorrect technique leads to inflammation, damage, and intense shoulder pain.

Vaccines Linked to SIRVA
SIRVA can occur with any vaccine administered via shoulder injection. This includes a wide range of vaccines, such as:
- Chickenpox (varicella) vaccines.
- Flu shots.
- Haemophilus influenzae type b (Hib) vaccines.
- Hepatitis A and B vaccines.
- Human papillomavirus (HPV) vaccines.
- Influenza vaccines.
- Measles, mumps, and rubella (MMR) vaccines.
- Meningococcal vaccines (MPSV4, MCV4).
- Pneumococcal conjugate and meningococcal vaccines.
- Rotavirus vaccines.
- Tetanus, diphtheria, and pertussis vaccines (DT, DTaP, Td, and Tdap).
Prevalence of SIRVA in the United States
The prevalence of SIRVA in the United States is generally considered rare, though exact numbers are challenging to determine. Studies focusing on the influenza vaccine suggest a rate of 1 to 2 cases per million. However, as the number of vaccinations, including seasonal influenza vaccines, continues to increase annually, it’s expected that SIRVA cases may also rise. A retrospective analysis involving nearly 3 million vaccinated individuals found an attributable risk of 7.78 additional cases of bursitis per million patients. Additionally, the number of reported adult SIRVA cases to the VICP increases each year.
SIRVA and Other Vaccine Injuries
The key distinction between SIRVA and other vaccine injuries lies in their location and administration method. SIRVA occurs when the vaccine is incorrectly injected into the shoulder, going too deep and causing pain and issues in the shoulder joint. Meanwhile, other vaccine injuries can occur due to various factors, such as allergies to vaccine components or manufacturing errors. They are related to how the body reacts to them. Preventing SIRVA is possible by ensuring the vaccine is injected correctly. However, preventing other vaccine-related injuries may be more complex, as they depend on factors like individual health and vaccine manufacturing.
Shoulder Injuries Associated with Improper Vaccine Administration
Mistakes during vaccine administration can result in various types of shoulder injuries. Here are common types of shoulder injuries linked to SIRVA:
- Brachial neuritis: Inflammation of the brachial plexus, causing severe shoulder pain, weakness, and numbness in the shoulder, arm, and hand.
- Deltoid bursitis: Inflammation of the bursa within the deltoid muscle, resulting in pain, swelling, and limited mobility in the shoulder.
- Frozen shoulder (adhesive capsulitis): Stiffness and pain in the shoulder joint due to inflammation and thickening of tissues surrounding the joint, leading to restricted movement.
- Impingement syndrome: Compression of the rotator cuff tendons between the bones of the shoulder joint, causing pain, inflammation, and reduced range of motion.
- Rotator cuff injuries: Tears or strains in the muscles and tendons of the rotator cuff, resulting in pain, weakness, and difficulty lifting or rotating the arm.
- Shoulder bursitis: Inflammation of the bursa in the shoulder joint, leading to pain, swelling, and limited mobility.
- Tendinitis: Inflammation of a tendon in the shoulder, causing pain and restricted movement.
These shoulder injuries can significantly impact daily life. If you experience persistent shoulder pain or mobility issues after vaccination, seek medical attention for evaluation and appropriate management.
Symptoms of SIRVA
Symptoms of SIRVA typically emerge shortly after vaccine administration, often within 48 hours or days. They can vary in severity and duration, but often include:
- Difficulty sleeping: Discomfort or pain in the shoulder that may worsen at night, affecting sleep quality.
- Limited range of motion: Difficulty moving the shoulder joint, such as raising the arm or reaching overhead.
- Pain with movement: Increased pain or discomfort when moving the shoulder, especially during certain activities.
- Shoulder pain: Sudden and intense pain in the shoulder area, which may be sharp, stabbing, or throbbing.
- Stiffness: Feeling of tightness or stiffness in the shoulder, making it challenging to move.
- Swelling and inflammation: Noticeable swelling, redness, or warmth around the shoulder joint.
- Tenderness: Sensitivity or tenderness to touch around the shoulder joint.
- Weakness: Reduced strength or muscle weakness in the shoulder and upper arm.
How Long Does It Take for SIRVA Symptoms to Appear?
Symptoms of SIRVA usually emerge within hours to days after receiving a vaccine. Many individuals may notice symptoms within the initial 48 hours following vaccination. The onset of SIRVA symptoms is often rapid, but the timing and severity can differ among individuals. Those affected may experience symptoms for months or even years.
Risk Factors for Developing SIRVA
Several factors can contribute to an individual’s risk of developing SIRVA:
- A smaller deltoid muscle: Difficulty in accurately targeting the muscle for injection may increase the risk of SIRVA in individuals with a smaller deltoid muscle.
- A thin physical build: Thinner individuals may have less muscle mass in the shoulder area, raising the risk of injecting into non-muscular tissues.
- Female sex: Differences in shoulder anatomy and muscle mass compared to males may put females at a higher risk of SIRVA.
- Improper injection techniques: Errors such as injecting too high on the arm can increase the risk of injecting into non-muscular tissues, leading to SIRVA.
- Individual anatomy: Variations in shoulder anatomy, such as shoulder width and muscle distribution, can influence SIRVA risk.
- Inexperienced healthcare providers: Lack of experience in proper injection techniques may increase the likelihood of errors leading to SIRVA.
- Injection site selection: Accurately identifying the injection site is crucial to preventing SIRVA.
- Needle length: Using a needle that is too long can result in injecting the vaccine too deeply into the shoulder, increasing the risk of SIRVA.
- Older age: Age-related changes in shoulder anatomy and muscle mass may heighten the risk of SIRVA in older individuals.
- Previous shoulder injury: A history of shoulder injuries or conditions may increase the susceptibility to SIRVA.
- Repeated vaccination: Receiving multiple vaccinations on the same shoulder within a short period may elevate the risk of SIRVA.
- Vaccine volume: Administering excessive vaccine volume can raise pressure within the shoulder joint, potentially causing damage and increasing SIRVA risk.
Diagnosing SIRVA
Diagnosing SIRVA typically involves several steps:
- Patient history: Gather details about recent vaccination history and shoulder symptoms onset to assess for potential SIRVA link.
- Physical examination: Conduct a thorough shoulder examination to check for signs of inflammation, tenderness, and limited motion.
- Review vaccine administration technique: Evaluate vaccine administration technique for deviations from guidelines that may indicate SIRVA.
- Imaging studies: Order ultrasound or MRI to confirm SIRVA by identifying structural abnormalities like inflammation or bursitis.
- Exclusion of other causes: Rule out alternative diagnoses like rotator cuff tears or adhesive capsulitis through careful assessment and testing.
By considering these factors, healthcare providers can accurately diagnose SIRVA, enabling prompt treatment and management for symptom relief and shoulder recovery.
Treatment Options for SIRVA
Treatment for SIRVA includes conservative and surgical approaches to relieve symptoms and promote recovery:
- Conservative treatments: Focus on non-invasive methods to reduce pain and inflammation, such as rest, pain relief medications, and steroid injections.
- Physical therapy: Guided exercises and stretches help improve shoulder function and reduce discomfort.
- Surgical intervention: Considered for severe cases where conservative treatments are ineffective. Surgery involves repairing damaged shoulder structures to address pain, weakness, and limited mobility.
Treatment for SIRVA is personalized based on symptoms and response to conservative measures. Seek medical evaluation if you experience persistent shoulder issues post-vaccination for proper management and recovery. Early intervention is crucial for optimal outcomes.
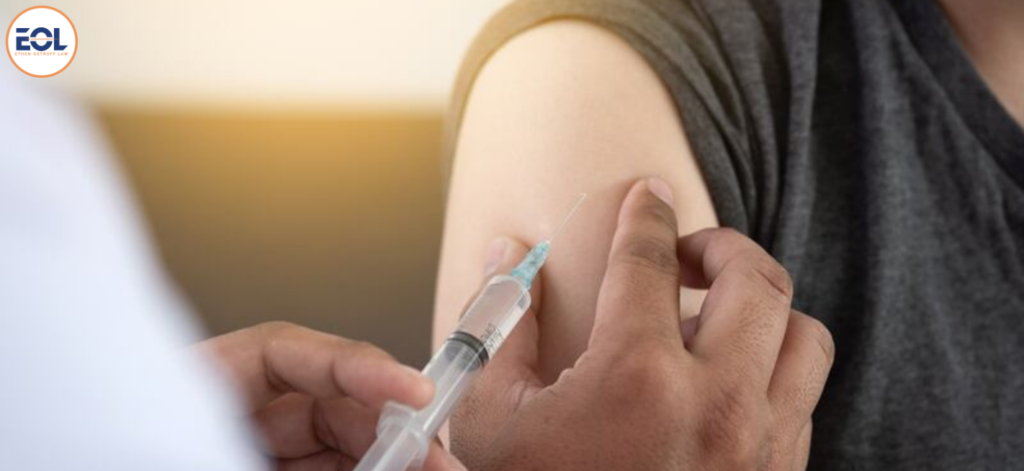
Does SIRVA Ever Go Away?
SIRVA can vary in how long it lasts. Sometimes, symptoms improve with treatment, while in other cases, they persist or become chronic. Recovery depends on factors like the injury itself, your health, and how well treatment works for you. Seeking prompt medical attention and following your healthcare provider’s advice can help manage symptoms and improve your chances of recovery.
Preventing SIRVA
To prevent SIRVA, healthcare providers can implement these strategies:
- Anatomy assessment: Assess shoulder anatomy before vaccination to identify any variations that may affect injection accuracy.
- Education and training: Stay updated on best practices through ongoing education and training in vaccine administration.
- Injection site landmarking: Mark the injection site accurately to target the deltoid muscle effectively.
- Needle length selection: Choose the appropriate needle length based on the patient’s body size to avoid deep injections.
- Patient education: Inform patients about SIRVA risks and encourage reporting any shoulder discomfort post-vaccination.
- Patient positioning: Ensure patients are positioned properly during vaccination to aid accurate needle placement.
- Proper injection technique: Train healthcare providers thoroughly in correct injection techniques to minimize SIRVA risk.
- Quality assurance measures: Implement quality checks to ensure adherence to vaccination guidelines and protocols.
By following these preventive measures and stressing accurate vaccine administration, healthcare providers can lower SIRVA incidence and enhance patient safety during vaccination.
Legal Implications of SIRVA
SIRVA has important legal implications for those affected. Here’s what you need to know:
- Legal liability: Healthcare providers must administer vaccines carefully. If negligence leads to SIRVA or similar injuries, they could be liable, and you can seek vaccine compensation.
- Product liability: Vaccine manufacturers might face legal action if their products cause SIRVA. You can file claims against them for compensation.
- Statute of limitations: In the US, you usually have three years from vaccination to file a claim with VICP. Some exceptions apply.
- VICP: In the US, if you have SIRVA or other vaccine-related injuries, you might get compensation through VICP. It covers medical costs, lost wages, and related expenses.
Understanding these aspects is crucial if you’re affected by SIRVA. Legal guidance from a SIRVA lawyer can help you navigate the process and seek compensation.
The National Vaccine Injury Compensation Program (VICP)
The National Vaccine Injury Compensation Program (VICP) was established in 1986 to provide financial assistance to people affected by covered childhood vaccines, including SIRVA. Its goals are to ensure a stable vaccine supply, control costs, and offer accessible compensation for vaccine-related injuries.
Administered by the Health Resources & Services Administration (HRSA) and overseen by the Office of Special Masters at the US Court of Federal Claims, VICP simplifies the process of resolving vaccine injury claims. HRSA reviews claims and advises the US Department of Justice, which represents the Secretary of Health and Human Services. The “vaccine court” or the Office of Special Masters decides on compensation eligibility. HRSA and the vaccine court work together to ensure fair compensation for individuals harmed by specific vaccines, including SIRVA. VICP proceedings happen only in the US Court of Federal Claims, without a jury.
Since its start in 1988, VICP has compensated individuals injured by covered vaccines, including SIRVA. Even without a formal finding, people can receive compensation through settlement agreements.
Vaccine Injury Table
The Vaccine Injury Table, crafted by the U.S. Department of Health and Human Services, is a guide listing vaccine, associated injuries, and the timeframe for symptoms’ onset. Updated regularly, it helps determine compensation eligibility under the VICP. This table specifies covered vaccines, eligible injuries, and expected symptom onset periods. In cases not on the table or outside the listed timeframe, the petitioner must demonstrate the vaccine’s role in causing the injury. The Vaccine Injury Table plays a crucial role in deciding compensation under the VICP, ensuring fairness and clarity in the process.
Vaccines Covered and Not Covered by the VICP
Here is a summary of the Vaccine Injury Table for claims filed on or after January 3, 2022, as listed on the HRSA’s website:
Covered vaccines and related injuries:
- Diphtheria, tetanus, pertussis (DTaP) vaccines. Anaphylaxis, encephalopathy, and shoulder injury related to vaccine administration (SIRVA)
- Haemophilus influenzae type b (Hib) vaccines. Anaphylaxis, encephalopathy, and SIRVA.
- Hepatitis A vaccines. Anaphylaxis and SIRVA.
- Hepatitis B vaccines. Anaphylaxis, Guillain-Barré Syndrome (GBS), and SIRVA.
- Human papillomavirus (HPV) vaccines. Anaphylaxis, GBS, and SIRVA.
- Influenza vaccines. Anaphylaxis, GBS, and SIRVA.
- Measles, mumps, rubella (MMR) vaccines. Anaphylaxis, encephalopathy, and SIRVA.
- Meningococcal vaccines. Anaphylaxis, GBS, and SIRVA.
- Pneumococcal conjugate vaccines. Anaphylaxis, encephalopathy, and SIRVA.
- Polio vaccines. Anaphylaxis, encephalopathy, and SIRVA.
- Rotavirus vaccines. Intussusception and SIRVA.
- Tetanus toxoid-containing vaccines. Anaphylaxis, encephalopathy, and SIRVA.
- Varicella (chickenpox) vaccines. Anaphylaxis, encephalopathy, and SIRVA.
Not covered vaccines and injuries:
- COVID-19 vaccines. Anaphylaxis, thrombosis with TTS, myocarditis, pericarditis, and VITT.
- Injuries or conditions not caused by the vaccine.
- Injuries or conditions not covered by the VICP.
- Injuries or conditions not listed in the Vaccine Injury Table.
For a complete list of vaccines and related injuries covered by the VICP, refer to the Vaccine Injury Table provided by HRSA.
Eligibility for SIRVA Compensation Under the VICP
To be eligible for compensation for SIRVA through VICP, you need to meet these criteria:
- Covered vaccine: The vaccine you received must be on the list of covered vaccines under VICP.
- Listed injury: SIRVA or the specific injury from vaccine administration must be listed on the Vaccine Injury Table.
- Timely symptoms: Symptoms of SIRVA must appear within a specific timeframe after vaccination, as specified in the Vaccine Injury Table.
- Duration or severity: The injury should last over six months or require hospitalization and surgery.
- File a petition: You must file a petition with the Office of Special Masters within the statute of limitations to seek compensation under the VICP for SIRVA.
Consult a healthcare provider or SIRVA attorney for detailed guidance on requirements.
Filing a SIRVA Claim with the VICP
If you’ve been diagnosed with SIRVA and want to claim compensation through VICP, follow these steps:
- Check the Vaccine Injury Table: Ensure SIRVA is listed as a recognized injury.
- Provide evidence: If SIRVA isn’t listed, you’ll need to prove the vaccine caused your injury.
- Submit a petition: File online or by mail with vaccine court, including vaccine details, symptoms, and medical treatment.
- Include medical records: Attach relevant documents supporting your SIRVA diagnosis and vaccine connection.
- Await special master assignment: Once submitted, a special master will review your case and may request more information.
- Attend a hearing: If eligible, a hearing will be scheduled to assess compensation based on injury severity and losses.
- Consider an appeal: If unsatisfied, you can appeal the decision to the U.S. Court of Federal Claims.
For detailed guidance on petition submission and navigating the VICP process, visit the U.S. Court of Federal Claims, Office of Special Masters, for comprehensive resources and assistance.
Common Reasons for VICP Claim Denials
VICP claims, including those related to SIRVA, may be denied for various reasons, including:
- Failure to establish a “severe” injury or illness.
- Failure to establish the timing of the first symptom, or “significant aggravation,” of injury.
- Failure to file a petition within the statute of limitations.
- Injury or condition not aligned with the time on the Vaccine Injury Table.
- Injury or condition not listed on the Vaccine Injury Table.
- Not meeting the technical requirements of the VICP.
- Not specifying the vaccine.
- Submitting insufficient evidence to support the claim.
- VICP does not cover the vaccine.
Appealing a Denied Vaccine Injury Claim
If your VICP claim is denied, follow these steps to appeal:
- Submit an appeal to the Court of Federal Claims within 30 days of the decision.
- A judge will review evidence from both parties.
- If the denial is upheld, you can appeal to the Court of Appeals for the Federal Circuit.
- Further appeals can be made to the U.S. Supreme Court if necessary.
Appealing a denied claim, including a SIRVA claim, under VICP can be complex. Seek advice from one of Ethen Ostroff Law‘s vaccine lawyers.
VICP Claim vs. SIRVA Lawsuit
When seeking compensation for a vaccine-related injury like SIRVA, individuals have two main options:
- VICP claim for SIRVA: This offers a no-fault alternative, streamlining the claims process. By petitioning the vaccine court, individuals diagnosed with SIRVA can seek compensation. It involves presenting evidence of the injury and attending a hearing to determine eligibility.
- Vaccine lawsuit for SIRVA: This is a legal action alleging vaccine-related harm, typically pursued in state or federal court if there’s disagreement with VICP’s decision or the need for additional damages.
Choosing between a VICP claim and a SIRVA lawsuit depends on the case specifics. Seek personalized advice from a vaccine injury attorney at Ethen Ostroff Law.
Can You Sue for SIRVA?
Yes, you can sue for SIRVA if you think you got it from a vaccine. It’s important to see a doctor first to confirm the injury. If your injury resulted from the vaccine being administered improperly, you might have a case. But there are specific legal procedures and programs for vaccine-related injuries, so consulting with a lawyer who knows this area of law is wise.
Should I Sue for SIRVA?
Deciding whether to sue for SIRVA is personal. Confirm the injury with a doctor, consult a SIRVA lawyer, and weigh your options. Consider your injury’s severity, proving negligence, and what you hope to achieve.
Types of Compensation Awarded Under the VICP
When seeking compensation for a vaccine-related injury like SIRVA through the VICP, individuals may receive various types of compensation:
- Lost wages. Covers both actual and expected lost wages due to the vaccine injury.
- Medical expenses. Covers past and future out-of-pocket medical costs, excluding insurance (except Medicaid).
- Pain and suffering. Awards for emotional distress associated with the vaccine-related injuries or conditions, with a maximum limit of $250,000 under VICP.
What Is the Average Payout for SIRVA?
The average amount of compensation awarded under the VICP for SIRVA can vary widely depending on the specific circumstances of each case. Factors such as the severity of the injury, the extent of lost wages and medical expenses, and the degree of pain and suffering experienced by the individual all play a role in determining the SIRVA compensation amount. Consult with a healthcare provider or a SIRVA attorney specializing in injury claims for detailed insights into vaccine injury settlements.
Negotiated Settlements in VICP Petitions
VICP petitions often lead to negotiated settlements for various reasons:
- Minimizing risk for both parties.
- Reducing time and litigation costs.
- Swift resolution of petitions.
- When the Department of Health and Human Services acknowledges vaccine causation, eliminating the need for court proceedings.
- When evidence from both sides supports a settlement.
These considerations aim to streamline the process and provide a quicker resolution for all involved parties.
How Long Do SIRVA Cases Take to Settle?
The time it takes for SIRVA cases to settle varies widely. Factors like case complexity, legal procedures, and whether a settlement is reached, or it goes to trial influence the duration. Some cases settle quickly through negotiation, while others may take months or even years.
Factors affecting the timeline include:
- Court backlog: Availability of court dates can affect scheduling.
- Legal procedures: Filing a SIRVA lawsuit, gathering evidence, and trial preparation can be time-consuming.
- Medical evidence: Collecting records and expert opinions on the injury’s extent can add to the duration.
- Settlement negotiations: Agreeing on terms may speed up resolution compared to going to trial.
Since each case is unique, there’s no set timeframe for settlement. It’s important to work closely with your SIRVA lawyer to understand the process and timeline specific to your situation.
Ethen Ostroff Law for VICP Claims or SIRVA Lawsuits
Vaccines are vital for preventing diseases, but SIRVA can happen. The VICP offers compensation for those affected. Navigating this process can be tough, so having a vaccine injury attorney is crucial. At Ethen Ostroff Law, our team understands the complexities of vaccine injury claims. We can help you file SIRVA claims through VICP or pursue a SIRVA lawsuit. We’ll assist in collecting medical records and represent you throughout the process. Contact us today for a free consultation.


